One Mole of Ammonium Nitrate Contains:
One most certainly would not. 4NH 4 3 PO 4 3PbNO 3 4 Pb 3 PO 4 4 12NH 4 NO 3.

Solved One Mole Of Ammonium Nitrate Contains O 3 Moles Of Chegg Com
The net charge is written with the magnitude before the sign.
. The chemical equation for this reaction is. C 1 mole of potassium. Copper is used for the absorption and used of iron in the formation of haemoglobin.
To safely make potassium nitrate put on gloves a gas mask and goggles and cut open a cold pack that contains ammonium nitrate. The mole is a unit for counting 602 x10 23 representative particles. Compute the ratio of the number of.
List the conversion factors used to convert between particles and moles. B 2 moles of sulfur. The chemical formula is NaClO and consists of one atom of sodium Na one atom of chlorine Cl and one atom of oxygen O.
Compute the difference in masses of one mole each of aluminium atoms and one mole of its ions. D 3 moles of potassium. NH 4 3 PO 4 3NH 3 H 3 PO 4.
Which one is heavier. One mole of potassium sulfate contains. Sodium hypochlorite is a strong liquid oxidizing agent and has a greenish or yellowish hue.
When 40 g of NaOH are present in one litre of NaOH solution the solution is known as normal N solution of NaOH. How many grams of Cu are. 2 How many grams of magnesium cyanide are needed to make 275 mL of a 0075 M solution.
Ammonium phosphate readily undergoes decomposition reaction emitting very toxic fumes. When writing the chemical formula for an ion its net charge is written in superscript immediately after the chemical structure for the moleculeatom. The solubility of halide salts increases from fluoride to iodide.
A solution of ThalliumIIINitrate 39g 100 mmol in 100ml methanol is added to a solution of alpha-methylstyrene 1182g 100mmol in 50ml methanol at room temperature with stirring and there is an immediate precipitation of ThalliumINitrate and after standing for 15 minutes the precipitate is filtered off washed with a little methanol and the filtrate is shaken for 5 min with. Ammonium phosphate reacts with lead nitrate forming lead phosphate and ammonium nitrate. Most ammonium salts are soluble and act as acids in liquid ammonia solutions.
Then dissolve 56 grams of potassium hydroxide in water and go outside to mix the potassium solution and purified. 5 What is the. Silver nitrate AgNO 3 is used to.
PbNO32aq 2 KClaq PbCl2s 2 KNO3aq What volume of a 100 M KCl solution must you add to 75 mL of a 02 M solution of PbNO32 to precipitate out all of the Pb2 ions. 4 Explain how to make one liter of a 125 molal sodium hydroxide solution. 3 How many grams of magnesium cyanide would you need to add to 275 mL of water to make a 0075 molal solution.
Find the mole fraction of the sodium chloride and of the water in the solution. A silver ornament of mass m gram is polished with gold equivalent to 1 of the mass of silver. When we carry out a chemical reaction using a solution of a salt such as ammonium dichromate we need to know the concentration of each ion present in the solution.
It is usually called bleach because it is the active ingredient in bleach. Calculate the molar mass of ammonium. One mole contains 602 x10 23 representative particles.
One mole of ammonium nitrite contains. A solution containing one gram equivalent weight of the solute dissolved per litre is called a normal solution. E none of the above.
A saturated solution of ammonium nitrate Divers solution named after Edward Divers contains 083 mol solute per mole of ammonia and has a vapour pressure of less than 1 bar even at 25 C 77 F. Mass of an electron is 91 x 10 -28 g. If an explosive contains 3450 g of ammonium nitrate how many mole of ammonium nitrate are present in the explosive.
Ammonium CO 3 2 carbonate H3O hydronium HCO 3 hydrogen carbonate bicarbonate OH hydroxide OCN cyanate CN cyanide SCN thiocyanate O2 2 peroxide N3 azide CrO 4 2 chromate NO2 nitrite Cr 2O7 2 dichromate NO3 nitrate MnO 4 permanganate NH2 amide SO4 2 sulfate ClO4 perchlorate SO 3 2 sulfite ClO3. Apply How does a chemist count the number of. If a solution contains 143 M NH 4 2 Cr 2 O 7 then the concentration of Cr 2 O 7 2 must also be 143 M because there is one Cr 2 O 7 2 ion per formula unit.
Explain how a mole is similar to a dozen. Ammonium Nitrate NH4NO3 is a main component of explosive mixtures used in mining quarrying and civil construction. That is a doubly charged cation is indicated as 2 instead of 2However the magnitude of the charge is omitted for singly charged moleculesatoms.
It forms phosphoric acid and ammonia. A 4 moles of oxygen. When potassium chloride KCl is added to a solution of lead nitrate PbNO32 a bright yellow precipitate is formed PbCl2.
The dozen is used to count 12 items. One molal solution contains one mole of the solute dissolved in 1000 g of the solvent. Mix the ammonium nitrate with hot water and pour the mixture through a coffee filter to purify it.
Uses of Ammonium phosphate NH. A 2 moles of nitrogen atoms B 4 moles of hydrogen atoms C 2 moles of oxygen atoms D All of A B and C E None of A B and C.
Solved Question 19 One Mole Of Ammonium Nitrate Contains 3 Chegg Com

Solved Question 19 One Mole Of Ammonium Nitrate Contains 3 Moles Of Hydrogen 2 Moles Of Oxygen 2 Moles Of Nitrogen 1 Mole Of Nitrogen None Of These Question 20 How Many Molecules
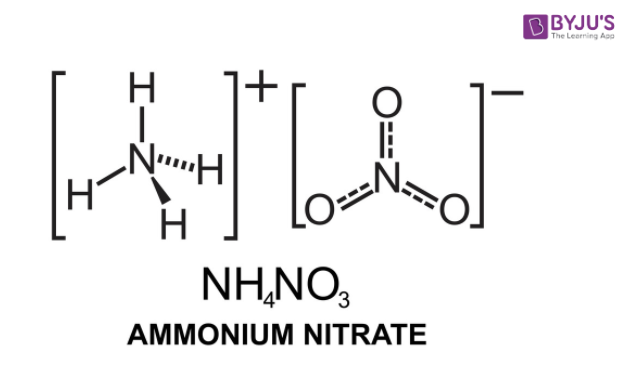
Ammonium Nitrate Nh Sub 4 Sub No Sub 3 Sub Structure Preparation Physical And Chemical Properties Uses With Faqs Of Ammonium Nitrate

Comments
Post a Comment